Mukuwedzera kune tekinoroji, iyo synthesis yeglycosides yagara iri yekufarira sainzi, sezvo iri maitiro akajairika muzvisikwa.Mapepa achangoburwa naSchmidt naToshima naTatsuta, pamwe chete nemanongedzero mazhinji anodudzwa imomo, akataura pamusoro pezvakasiyana-siyana zvinogoneka zvekugadzira.
Mukuumbwa kweglycosides, huwandu hweshuga hunosanganiswa nenyucleophiles, senge madoro, makabhohaidhiretsi, kana mapuroteni, kana sarudzo yakasarudzika neimwe yemapoka ehydroxyl ecarbohydrate inodiwa, mamwe mabasa ese anofanirwa kuchengetedzwa. danho rokutanga.Muchidimbu, enzymatic kana microbial process, nekuda kwekusarudza kwavo, inogona kutsiva yakaoma makemikari ekudzivirira uye deprotection matanho ekusarudza kubva kune glycosides mumatunhu.Zvisinei, nekuda kwenhoroondo yakareba yealkyl glycosides, kushandiswa kwema enzymes mukugadzirwa kweglycosides hakuna kuongororwa zvakanyanya uye kushandiswa.
Nekuda kwekugona kweakakodzera maenzayimu masisitimu uye mitengo yakakwira yekugadzira, enzymatic synthesis yealkyl polyglycosides haina kugadzirira kukwidziridzwa kusvika kumaindasitiri, uye nzira dzemakemikari dzinofarirwa.
Muna 1870, MAcolley yakashuma kuumbwa kwe "acetochlorhydrose" (1, mufananidzo2) nekuita kwe dextrose (glucose) ine acetyl chloride, iyo yakazotungamira kunhoroondo yeglycoside synthesis nzira.
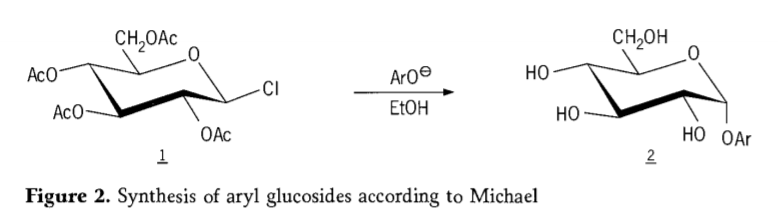
Tetra-0-acetyl-glucopyranosyl halides (acetohaloglucoses) yakazoonekwa kuti inobatsira pakati peiyo stereoselective synthesis ye pure alkyl glucosides.Muna 1879, Arthur Michael akabudirira mukugadzira aryl glycosides yakanyatsojeka, kubva kuColley's intermediates uye phenolates.(Aro-,Mufananidzo 2).
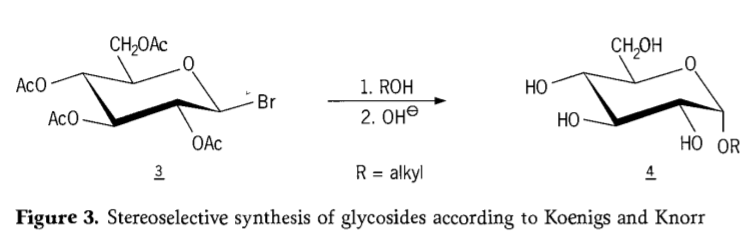
Muna 1901, kugadzirwa kwaMichael kune huwandu hwakawanda hwemakabhohaidhiretsi uye hydroxylic aglycons, apo W.Koenigs naE.Kuita kunosanganisira SN2 inotsiva pane anomeric kabhoni uye inopfuurira stereoselectively nekushandurwa kwekugadzirisa, ichigadzira semuenzaniso α-glucoside 4 kubva ku β-anomer yeaceobromoglucose yepakati 3. Koenigs-Knorr synthesis inoitika pamberi pesirivha kana mercury vanokurudzira.
Muna 1893, Emil Fischer akakurudzira nzira yakasiyana yakasiyana yekugadzirwa kwealkyl glucosides.Iyi nzira ikozvino inozivikanwa se "Fischer glycosidation" uye inosanganisira acid-catalyzed reaction ye glycoses nedoro.Chero nhoroondo yenhoroondo inofanirawo kusanganisira kuedza kwaA.Gautier kwekutanga muna 1874, kushandura dextrose neanhydrous ethanol pamberi pehydrochloric acid.Nekuda kwekukanganisa kwekutanga kuongorora, Gautier akatenda kuti aive awana "diglucose".Fischer akazoratidza kuti Gautier's "diglucose" yaive kunyanya ethyl glucoside (Mufananidzo 4).
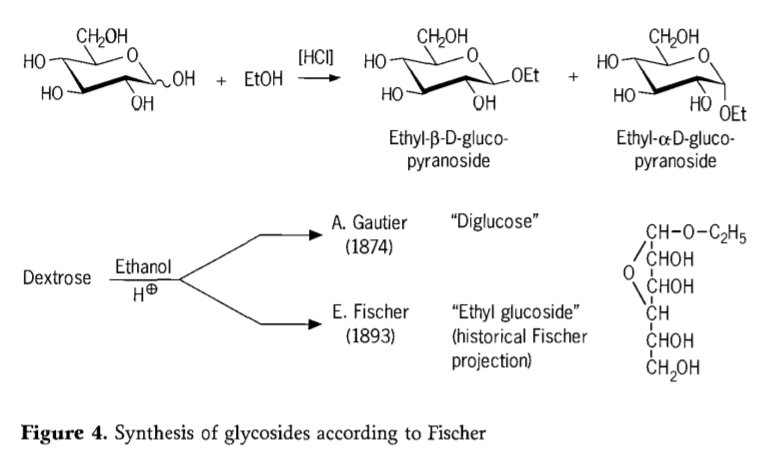
Fischer akatsanangura chimiro che ethyl glucoside nemazvo, sezvinogona kuonekwa kubva munhoroondo furanosidic formula yakarongwa.Muchokwadi, zvigadzirwa zveFischer glycosidation zvakaoma, zvakanyanya kuenzana musanganiswa wealpha/β-anomers uye pyranoside/furanoside isomers iyo inosanganisirawo zvisina kurongeka yakabatana glycoside oligomers.
Saizvozvo, ega ega mamorekuru emhando hazvisi nyore kuzviparadzanisa kubva kuFischer reaction musanganiswa, iro rave dambudziko rakakura munguva yakapfuura.Mushure mekuvandudzwa kweiyi nzira yekubatanidza, Fischer akazotora iyo Koenigs-Knorr synthesis yekuferefeta kwake.Tichishandisa maitiro aya, E.Fischer naB.Helferich ndivo vakatanga t kushuma kuumbwa kweketani refu alkyl glucoside ichiratidza surfactant properties muna 1911.
Kare muna 1893, Fischer akange anyatsoona zvakakosha zvealkyl glycosides, senge kugadzikana kwavo kwepamusoro kune oxidation uye hydrolysis, kunyanya mune yakasimba alkaline media.Maitiro ese ari maviri akakosha kune alkyl polyglycosides mune surfactant application.
Tsvagiridzo ine chekuita neiyo glycosidation reaction ichiri kuenderera mberi uye nzira dzinoverengeka dzinonakidza dze glycosides dzakagadzirwa munguva pfupi yapfuura.Mamwe maitiro ekuumbwa kweglycosides akapfupikiswa muMufananidzo 5.
Kazhinji, makemikari glycosidation maitiro anogona kukamurwa kuita maitiro anotungamira kune yakaoma oligomer equilibria mu acid-catalysed glycosyl exchange.
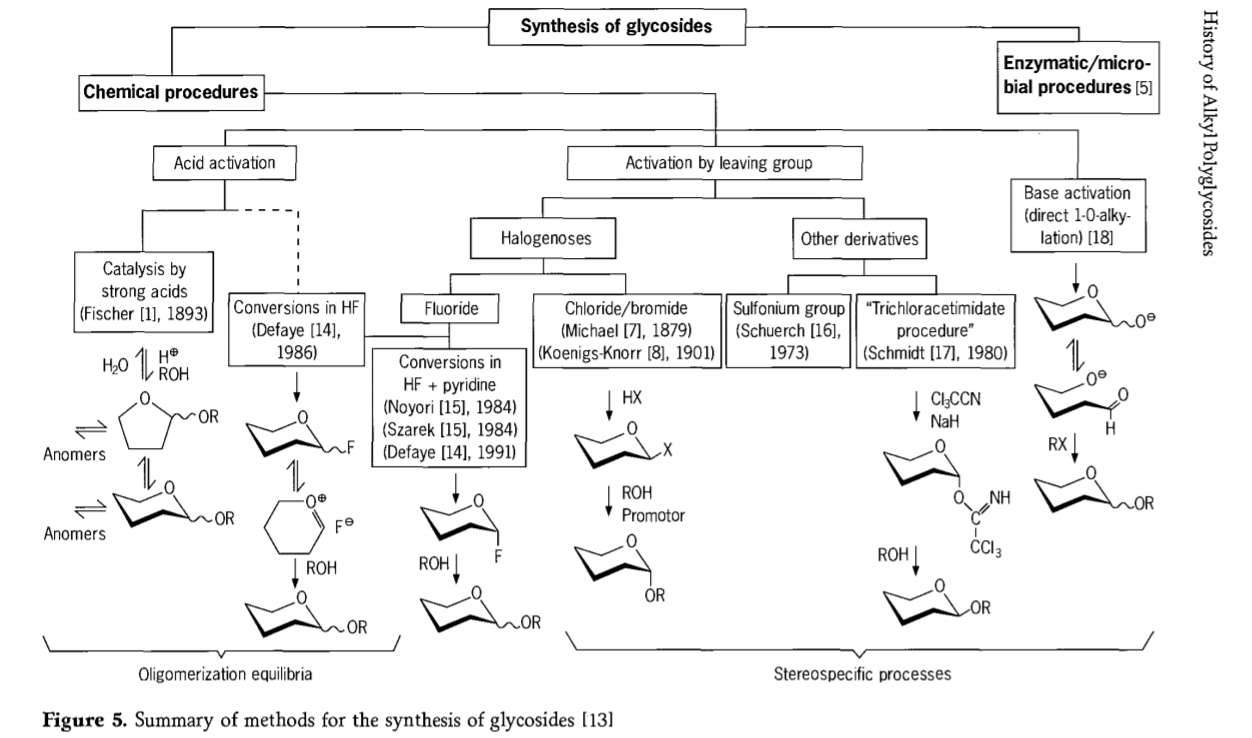
Magadzirirwo pane zvakafanira activated carbohydrate substrates(Fischer glycosidic reactions uye hydrogen fluoride(HF) maitiro ane asina kudzivirirwa carbohydrate mamorekuru) uye kinetics inodzorwa, isingadzokerike, uye kunyanya stereotaxic inotsiva maitiro.Rudzi rwechipiri rwemaitiro runogona kutungamirira mukuumbwa kwemarudzi ega ega kwete mumisanganiswa yakaoma yemaitiro, kunyanya kana yasanganiswa nemaitiro eboka rekuchengetedza.Makabhohaidhiretsi anogona kusiya mapoka pane ectopic kabhoni, senge halogen maatomu, sulfonyls, kana trichloroacetimidate mapoka, kana kuvhurwa nemabhesi asati atendeuka kuita triflate esters.
Munyaya yeglycosidations muhydrogen fluoride kana mumisanganiswa yehydrogen fluoride uye pyridine (pyridinium poly [hydrogen fluoride]), glycosyl fluorides inoumbwa in situ uye inoshandurwa zvakanaka kuita glycosides, semuenzaniso nemadoro.Hydrogen fluoride yakaratidzwa kuva yakasimba activating, nondegrading reaction medium;equilibrium auto condensation (oligomerization) inoonekwa yakafanana neiyo Fischer maitiro, kunyangwe maitiro ekuita angangove akasiyana.
Kemikari yakachena alkyl glycosides yakakodzera chete kune yakakosha maapplication.Semuyenzaniso, alkyl glycosides yakashandiswa zvinobudirira mukutsvakurudza kwebiochemical yekristallisation ye membrane proteins, senge matatu dimensional crystallization ye porin uye bacteriorhodopsin pamberi pe octyl β-D-glucopyranoside (zvimwe zviedzo zvinoenderana nebasa iri zvinotungamira kuNobel. mubairo muchemistry yeDeisenhofer, Huber naMichel muna 1988).
Munguva yekuvandudzwa kwealkyl polyglycosides, nzira dzestereoselective dzakashandiswa pachiyero cherabhoritari kugadzira zvinhu zvakasiyana-siyana zvemuenzaniso uye kudzidza physicochemical properties, nekuda kwekuoma kwavo, kusagadzikana kwepakati uye huwandu uye maitiro akaoma ekugadzirisa. wasters, syntheses yerudzi rweKoenigs-Knorr nedzimwe nzira dzekudzivirira dzeboka zvingagadzira matambudziko makuru ehunyanzvi uye ehupfumi.Maitiro emhando yeFischer haana kuomesesa uye ari nyore kuita pachiyero chekutengesa uye nekudaro, ndiyo nzira inosarudzika yekugadzirwa kwealkyl polyglycosides pamwero mukuru.
Nguva yekutumira: Sep-12-2020





